SYNCRONYS Newsletter – March 2022
SYNCRONYS eNewsletter
The delivery of healthcare is transforming into a patient-centered and value-based system, which requires changes in the sharing of health information to enhance the patient’s experience, improve health outcomes, and reduce costs (“The Triple Aim”).
A Health Information Exchange (HIE) is critical to this transformation for a variety of health care stakeholders (clinicians, laboratories, hospital, pharmacy, health plans, payers, and patients). SYNCRONYS, the designated HIE for the State of New Mexico, understands the importance of continuously evolving the heath information sharing ecosystem to engage all stakeholders successfully and sustainably in the process of health information sharing.
SYNCRONYS – 2021 in review
SYNCRONYS had a productive year in 2021 which included completing all 42 deliverables required in the HSD health interoperability program contract. SYNCRONYS effectively brought together several vendor partners to create a seamless interoperability solution for New Mexico through the HIE. New high value use cases were added and included diagnostic imaging sharing, Substance Use Disorder, Mental Health Coordination, Transition of Care, Expanded ED insights and decision support, Hepatitis C management, Advanced Directive/MOST registry and Advanced Analytics. SYNCRONYS worked with the New Mexico Legislators to successfully amend the New Mexico Electronic Medical Records Act to align state privacy regulations with the more stringent federal HIPAA regulations. The change in the EMRA allowed for a change in workflow for HIE users to no longer require consent in advance when viewing a record in the HIE for treatment, payment or operations (TPO). SYNCRONYS supported the New Mexico Department of Health during the COVID19 pandemic by integrating 30+ new laboratories performing COVID testingto support the daily COVID testing counts and provided HIE access to the DOH contract tracers.
Future vision, SYNCRONYS is looking ahead to 2022 and beyond in three key areas.
- Infrastructure in place in 2022 to support the Office of the National Coordinator (ONC) future vision for the Trusted Exchange Framework and Common Agreement (TEFCA) and Qualified Health Information Network (QHINs) as they are adopted.
- As a member of Civitas Networks for Health, SYNCRONYS is supporting Civitas’s future vision of Health Data Utility (HDU) to advance clinical and public health use cases. SYNCRONYS’ structure is aligned with the HDU Model and is well positioned to adopt future use cases.
- SYNCRONYS will continue to expand the quality and quantity of health data, participation of HIE subscribers and add additional high value functionality.
Use Cases
Hepatitis-C
In follow-up to our article in the December 2021, eNewsletter, SYNCRONYS, in collaboration with the Rhodes Group, released a clinical decision support tool to align with the New Mexico Health and Human Services Hepatitis C Checklist. The goal is to improve efficiency of Hepatitis C diagnosis, treatment initiation and monitoring. This monthly report aggregates a variety of data sources to measure the tool’s use and outcomes.
Number of Patients with an HCV Infection
SYNCRONYS and Rhodes Group measure, in near real-time, the number of patients with the clinical definition of an HCV infection by assessing lab results indicative of the clinical diagnosis of Hepatitis C. Table 1 conveys the total number of patients whose latest Hepatitis C quantitative test result indicates the patient is infected with Hepatitis C.
Figure 1. Total Number of Patients per Month with Hepatitis C in SYNCRONYS’ Dataset.
| Month | Total No. of Infections |
| 8/31/2021 | 12,248 |
| 9/30/2021 | 11,574 |
| 10/31/2021 | 11,388 |
| 11/30/2021 | 11,366 |
| 12/31/2022 | 11,248 |
| 1/31/2022 | 11,182 |
| 2/28/2022 | 11,313 |
The table indicates, since the Fall season of 2021, a slight decline in the number of patients. Between August 2021 and February 2022, a 7.6% decrease in patients with Hepatitis C was observed. By contrary, a 1.2% increase in total infected patients was observed in February 2022.
HCV Tool Use
Released in mid-October, the HCV Summary Tool aggregates, reviews, and displays the most current information needed enabling users to understand a patient’s status pertaining to their infection. From October 19, 2021, to January 19, 2022, the tool was used to assess 179 patients. Since January 19, 2022, the tool has been used to assess 236 patients, a 31.8% increase within half the amount of time (Figure 2).
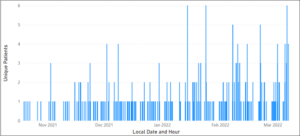 Figure 2. Number of patients the HCV Summary Tool was clicked on by SYNCRONYS users since inception.
Figure 2. Number of patients the HCV Summary Tool was clicked on by SYNCRONYS users since inception.
SYNCRONYS has performed significant outreach over the last twelve months with a focus on clinics who may benefit from using the HCV Summary Tool and Figure 1 confirms the success. With end-users increasing and number of patients with HCV decreasing, SYNCRONYS and Rhodes assessed the total number of patients achieving a sustained viral load.
Number of Patients Achieving a Sustained Viral Load
Sustained virologic response (SVR) is indicative of not detecting the hepatitis C virus twelve weeks or more after a patient has completed treatment. Most patients who receive this result are considered cured. Measuring and reporting this data point from within the SYNCRONYS dataset is important for the state of New Mexico, thus SYNCRONYS and Rhodes assessed the number of patients who achieved SVR since July 2021 (Figure 3).
Although the number of patients achieving SVR has been declining since July 2021, there has been a 30% increase in SVR measurements in 2022 compared to December 2021.
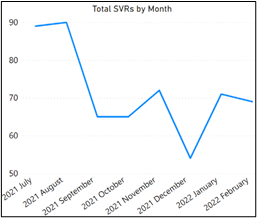 Figure 3. Total SVRs by Month
Figure 3. Total SVRs by Month
This data point, when aligned with use of the HCV Summary Tool, might indicate clinical users are becoming more effective in monitoring HCV. SYNCRONYS and Rhodes Group will continue to review these numbers and begin outreach to users confirming its effectiveness.
Population Health Analytics and Risk Assessment Solutions
SYNCRONYS’ population health analytics and risk assessment solutions provide the ability for clinicians, in both inpatient and outpatient settings, to review critical clinical information across the population. Identifying key areas of risk and opportunity based on cost, ED and inpatient utilization, mortality, acute events, and chronic conditions by specific diagnosis or diagnosis group, for the present timeframe (compared to KPIs if provided) and for future risk. The population, event, condition, and individual risk stratification is based on clinical data, utilization, social determinants of health as well as claims data. The future risk can be assessed for the general population or specific populations, defined by multiple filters, combinations of events and/or conditions, and/or transition risk of an inpatient or ED readmission. The risk level is based on the risk level in 30 and/or 90 days and identifies when the risk level has changed. The analytics also provides filters to identify patients with new diagnoses. All analytics are available to drill down to a list of targeted patients and then into the individual patient profile with multiple variables providing a weight of importance to each variable.
The Hospital Performance Reports and Cost and Utilization Performance Reports allows the clinician to focus on specific variables. For example, the Hospital Performance Report provides statistics vs KPIs for the last 12 months for inpatient (IP) 30-day readmission rate, IP average length of stay, IP mortality rate, IP sepsis rate, ED 30-day readmission rate, and ED average length of stay (ALOS). Multiple filters such as targeted diagnoses are available to refine the search, and the opportunity costs all are risk adjusted by the individual patient to account for sicker patients.
Multiple filters are available to easily refine the search for data and analytical insights which allow clinicians to focus on their health care priorities and accurately identify the best targets (patients, conditions, providers) for improving overall patient health and decreasing inappropriate utilization. Doctors, nurses, other clinicians use this to design, review outcomes, and improve outreach/care management/ population health programs in all settings.
News around the nation.
Medicare Promoting Interoperability Program 2022
The information below is taken from the CMS webinar on December 2, 2021
The following highlights information from CMS on updates on the EHR reporting requirements, certified EHR technology requirements, electronic clinical quality measured changed and objectives, measures and scoring requirements.
As of January 1, 2022, the previously known EHR Incentive Programs, Medicare and Medicaid Promoting Interoperability Programs, and Medicaid Promoting Interoperability Programs, are now officially the Medicare Promoting Interoperability Program. We felt it beneficial to our readers to highlight some of those changes occurring in 2022.
- EHR Reporting Period:
- A minimum of any continuous 90-days for eligible hospitals and CAHs
- Electronic Prescribing Objective:
- Available bonus points for PDMP will increase from 5 points to 10 points
- Health Information Exchange Objective:
- Health Information Exchange Bi-Directional Exchange measure (worth 40 points) added as an alternative to the 2 existing measures
- Public Health and Clinical Data Exchange Objective:
- Requiring reporting a “yes” on 4 of the public health and Clinical Data Exchange Objective measures, worth up to 10 points;
- Public Health Registry Reporting and Clinical Data Registry Reporting measures will remain optional and available for a total of 5 bonus points.
- Scoring Threshold:
- Increasing the minimum scoring threshold from 50 points to 60 points
- Attestation:
- Requiring eligible hospitals and CAHs to attest to having completed an annual self-assessment of the SAFER Guides measure, under the Protect Patient Health Information objective, beginning with the CY 2022 EHR reporting period.
- Removing attestation statements 2 ad 3 from the Medicare Promoting Interoperabilit Program’s prevention of information blocking requirement.
- eCQMs:
- Safe use of Opiods eCQM is now mandatory for CY 2022 reporting (optional in 2021)
- Requiring reporting a “yes” on 4 of the public health and Clinical Data Exchange Objective measures, worth up to 10 points;
The 2022 EHR Reporting period timeline:
| Reporting Year
2022 |
Attestation
2/28/23 |
Hardship Exception 2023 | Payment Adjustments 2022-2024 |
| Begins 1/1/22 | Final day to attest using Quality Net | Eligible hospitals and CAHs who did not demonstrate meaningful use can submit a Hardship Exception Application no later than September 1 | Eligible hospital FY 2024 |
| Ends 12/31/22 | CAHs 2022 |
Eligible hospitals and CAHs must earn a minimum of 60 out of 100 possible points to be considered a meaningful user. As a meaningful ser, you avoid the downward payment adjustments. There are opportunities to earn up to 15 additional bonus points. It is a requirement that participants report on all required measures, regardless of final sores, to be considered a meaningful user. If you skip any of the requirements (even scoring above 60 points), you are no longer a meaningful user.
Here is a look at the Health Information Exchange (HIE) Objective Overview & Measures
Support Electronic Referral Loops by Sending Health Information:
|
Support Electronic Referral Loops by Receiving and Reconciling Health Information:
|
OR | Bi-Directional Exchange through Health Information Exchange (HIE): (Alternative to two previous HIE measures)
|
Under the public health and clinical data exchange objective, an eligible hospital or CAH is considered in one of the levels of active engagement with a public health agency by reporting on four measures worth up to 10 points with exclusions available.
By exchanging data and subscribing to SYNCRONYS, the state of New Mexico’s HIE, 50 of the 60 points required would be achieved by participating hospitals.
To obtain additional detail, and information including scoring methodology, CEHRT requirements, eCQM requirements, electronic prescribing objectives and measures, public health and clinical data exchange objective and measures, security risk analysis measure, SAFER guides, and to understand actions to limit or restrict the compatibility or interoperability of CEHRT, visit the CMS website at CMS.gov.
As we send out our first eNewsletter of 2022, the SYNCRONYS Team would like to thank you for your support and business. If you would like more information about items in our newsletter or any other solution SYNCRONYS provides, please contact us at info@syncronys.org, and our website is always open at syncronys.org.